Introduction
Anti-CD19 chimeric antigen receptor-modified T cell therapies (CART19) have revolutionized treatment of patients (pts) with relapsed/refractory (R/R) diffuse large B cell lymphoma (DLBCL) and high grade B cell lymphoma (HGBL). However, progression of disease following receipt of CART19 is common with only approximately one-third of pts achieving long-term remission in the standard-of-care setting (Transplant Cell Ther. 2022 Sep;28(9):581.e1-581.e8, Blood (2022) 140 (Supplement 1): 1584-1587). While loss of CD19 antigen expression antigen is one mechanism of CART19 failure, other tumor-intrinsic factors which may result in CART19 failure remain poorly defined. Clinical laboratory mutation analysis (CLMA) performed on tumor specimens can identify recurring genetic mutations which may predict for clinical outcomes; however, the predictive value of CMLA has not been well-established for DLBCL/HGBL pts treated with CART19. Here, we analyze the impact of genetic mutations as detected by CLMA, along with other clinicopathologic characteristics, on survival outcomes of DLBCL/HGBL pts following receipt of CART19.
Methods
We collected data from 46 pts with DLBCL or HGBL treated at the University of Pennsylvania between 2018 and 2021. Patient tumors were sequenced with one of three sequencing panels comprised of 41-116 genes prior to receiving commercial CART19. Cox proportional hazards regression was used to assess the impact of clinicopathologic characteristics on disease progression and death, with characteristics demonstrating p <0.10 on univariate analysis included in multivariate analysis. Estimated progression free survival (PFS) and overall survival (OS) from the time of CART19 infusion were calculated using Kaplan-Meier estimates.
Results
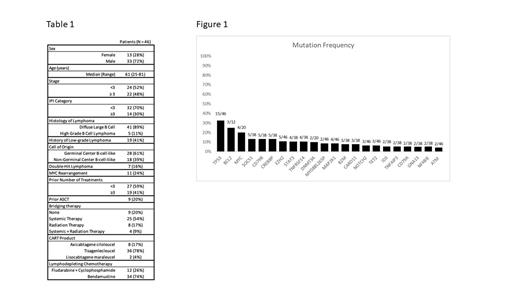
Baseline clinicopathologic characteristics are listed in Table 1. A total of 30 of 46 pts had at least one tumor mutation detected prior to receiving CART19, with 22 genes mutated in at least two pts (Figure 1). The most frequent mutations were TP53 (15 of 46, 32.7%), BCL2 (3 of 12, 25%), MYC (4 of 20, 20%), SOCS1 (5 of 38, 13.2%), CD79B (5 of 38, 13.2%), CREBBP (5 of 38, 13.2%), EZH2 (5 of 46, 10.9%), STAT3 (4 of 38, 10.5%), TNFRSF14 (4 of 38, 10.5%) and DNMT3A (2 of 20, 10%). The median length of follow-up from the time of CART19 infusion was 29.6 months. For all pts, the rates of 2 year (y) PFS and OS were 21% (95% confidence interval [CI] 10-34%) and 56% (95% CI 40-69%), respectively. Univariate analysis of mutations present in ≥10% of cases revealed that TNFRSF14 mutations predicted for lower risk of disease progression at 2y (HR 0.16, 95% CI 0.02-1.186, p = 0.07). No other mutation was associated with either disease progression or death at 2y. Univariate analysis of baseline clinicopathologic characteristics also identified International Prognostic Index (IPI) score ≥3 at the time of relapse preceding CART19, HGBL classification, no history of indolent lymphoma (IL), MYC rearrangement (-R) and double hit lymphoma (DHL) as predictive of disease progression at 2y; however, none of these characteristics remained predictive on multivariate analysis. Additionally, IPI score ≥3 at the time of relapse preceding CART19, HGBL classification, no history of IL, MYC-R and DHL were also predictive of death at 2y on univariate analysis; however, none of these characteristics remained predictive on multivariate analysis.
Conclusions
Tumor characteristics, including recurring genetic mutations as detected by CLMA, may not independently predict for survival outcomes for DLBCL/HGBL pts following receipt of CART19. However, our finding that TNFRSF14 mutations predict for a lower rate of disease progression at 2y on univariate analysis may be explained by loss of the inhibitory effect of HVEM on T helper cells through binding of B and T lymphocyte attenuator (BTLA) (Cell. 2016 Oct 6;167(2):405-418.e13). Additionally, our finding that TP53 mutations did not predict for inferior PFS following receipt of CART19 is in agreement with that of a prior analysis (J Clin Oncol. 2022 Feb 1;40(4):369-381). Performance of CLMA on a larger number of tumor samples from DLBCL/HGBL pts subsequently treated with CART19, which is planned, may be informative.
Disclosures
Schuster:Celgene: Consultancy, Research Funding; Nanovecter: Consultancy; DTRM: Research Funding; Juno Therapeutics: Research Funding; Incyte: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Janssen: Consultancy; Legend Biotech: Consultancy; Loxo: Consultancy; Merck: Research Funding; Pharmacyclics: Consultancy; BiGene: Consultancy; Acerta: Consultancy; MustangBio: Consultancy; Abbvie: Research Funding; Adaptive Biotechnologies: Research Funding; TG Therapeutics: Research Funding; Morphosys: Consultancy; Nordic: Consultancy; Regeneron: Consultancy; Novartis: Consultancy, Research Funding. Nasta:Millennium Takeda: Research Funding; ADC Therapeutics: Honoraria; Merck-Data Safety Monitoring Committee: Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceutical: Research Funding; Loxo/Lilly: Research Funding; Genentech/Roche: Research Funding; Pharmacyclics: Research Funding; Accrotech: Honoraria; Raphael: Research Funding. Svoboda:Incyte: Consultancy, Research Funding; TG Therapeutics: Research Funding; Merck: Research Funding; ADCT: Consultancy; Adaptive: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Atara: Consultancy; BMS: Consultancy, Research Funding; Genmab: Consultancy; Pharmacyclics: Consultancy, Research Funding; SEAGEN: Consultancy, Research Funding. Barta:Daiichi Sankyo: Consultancy; Affimed: Consultancy; Janssen: Consultancy; Acrotech: Consultancy. Chong:Novartis: Honoraria; Abbvie: Research Funding; Genentech: Research Funding; MJH Healthcare Holdings, LLC: Honoraria; BMS: Honoraria; Beigene: Honoraria. Ruella:GlaxoSmithKline: Consultancy; NanoString: Consultancy, Research Funding; viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; Beckman Coulter: Research Funding; Bristol Myers Squibb: Consultancy; AbClon: Consultancy, Research Funding; Bayer: Consultancy. Landsburg:Karyopharm: Membership on an entity's Board of Directors or advisory committees; ADCT: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Membership on an entity's Board of Directors or advisory committees; Calithera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Curis: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel funding; Morphosys: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal